| Table of Contents |  |
|
Original Article
| ||||||
| Differences in the energy of DNA strands interaction in the near start-codon sequences of three bacterial genomes | ||||||
| Papirny Maxim A.1, Pasiuga Vladimir N.2, Shckorbatov Yuriy G.3 | ||||||
|
1Post-graduate student, National Scientific Centre "ISSAR" NAAS, Kharkiv, Ukraine.
2Researcher, Research Institute of Biology, V. N. Karazin Kharkiv National University, Kharkiv, Ukraine. 3Head of Department of Genetics, Research Institute of Biology, V. N. Karazin Kharkiv National University, Kharkiv, Ukraine. | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] |
| How to cite this article |
| Papirny MA, Pasiuga VN, Shckorbatov YG. Differences in the energy of DNA strands interaction in the near start-codon sequences of three bacterial genomes. Edorium J Bioinforma 2015;1:1–8. |
|
Abstract
|
|
Aims:
The energy of DNA strands interaction (DSI) can regulate binding of enzymes and regulatory proteins to DNA in gene promoter and upstream region. The purpose of this work was to investigate the mean value of the energy DSI in the near promoter zone (± 200 nucleotides) of three bacterial genomes.
Methods: We used the computer analysis for assessment of the energy of DSI in 90 in direct orientation and 90 genes in reverse orientation for every genome in proximity of start codon for E. coli, B. subtilis, and S. typhimurium genomes. Results: The DSI energy in genes of three tested bacterial species decreases in the prepromoter zone and comes to the elevated level in the coding zone of gene. The assessed mean energy of DSI differs in all tested species. It is higher (in absolute value) in genes of E. coli and S. typhimurium than in genes of B. subtilis in the prepromoter and promoter zone (area from -200 to -1). The DSI energy in the promoter and prepromoter zones (from -150 to -1 nucleotides) and in coding zone (from + 51 to + 150 nucleotides) is lower in genes of S. typhimurium and B. subtilis with reverse orientation than in directly oriented genes. In genes of E. coli such regularity was not observed. Conclusion: The proposed approach- the assessment of the DSI energy in the genomes is a perspective tool for determination differences in genotype between the organisms of different systematic position. For instance, the observed similarity in DSI in the near promoter zone between E. coli and S. typhimurium and difference in DSI to B. subtilis may be connected with the relative systematic proximity between E. coli and S. typhimurium and relative systematic distance of B. subtilis from these species. | |
|
Keywords:
Bacillus subtilis, DNA strands interaction energy, Escherichia coli, Gene activity regulation, Gene orientation, Salmonella typhimurium
| |
|
Introduction
| ||||||
|
The physical properties of DNA promoter sequences namely the energy of interaction of DNA strands in double helix is very important for regulation of gene activity [1]. The definite sites in bacterial promoter have very conservative composition connected with their specific function. Stress-induced DNA duplex destabilization is closely associated with specific promoter regions [2]. The -10 motifs binds the transcription initiation complex, while the -35 motif participate in regulation of transcription activity of promoters. In the region around position -50 is located A+T rich UP element [3]. Most of the promoter prediction programs search sequences for conserved -10 motifs, and in some cases also include -35 motifs [4] [5]. The ability to DNA melting in the promoter zone is regarded as very important characteristics that influence on gene activity [6]. The theoretical model proposed by Peyrard, Bishop, and Dauxois [7], accurately describes experiments with DNA denaturation, and the formation and role of bubble states in the premelting regime [8]. There are differences in nucleotide composition between genes in direct and reverse orientation. Leading and lagging strands of DNA are asymmetric in nucleotide contents. In the broad study of bacterial genomes of different species done by Lobry and Sueoka was demonstrated that most of the 43 chromosomes taken under consideration were relatively enriched in G over C and in T over A and slightly depleted in G+C in the leading strand compared with the lagging strand [9]. The problems of strand asymmetry are analyzed in many reviews [9] [10] [11] [12] [13] [14]. In bacterial genomes some of genes are located in the leading strand, the direction of their transcription is the same as direction of replication (directly oriented genes) and some of genes are located in the lagging strand (genes with reverse orientation) [13]. The frequency of leading strand genes is ~75 % in B. subtilis [15], but only ~55 % in E. coli genome [16]. Such differences in gene location may be connected with RNA polymerase properties of these species. In B. subtilis there are two different RNA polymerases for leading and lagging strands [17]. In E. coli the two core polymerases are not pre-dedicated to one strand and can be interchanged [18]. As known the promoter region enriched with AT-nucleotide which enables linkage RNA-synthesizing machinery. In our previous investigation, it was demonstrated that promoters with minimal and maximal energy have low activity (strengths), and the strongest promoters are characterized by intermediate mean energy of double strand interaction (DSI) values [19]. The minimum of DSI is applied as a parameter for search of promoters in DNA sequences [19]. In connection with the mentioned above findings we studied the energy of DSI in the promoter and near-promoter area in genes with direct and reverse orientation of E. coli, S. typhimurium, and B. subtilis [20]. Two of bacterial species - E. coli and S. typhimurium are evolutionary closer, and the other species - B. subtilis located more distantly from these in evolutionary sense. The systematic position and evolutionary connections between the species analysed in this work are presented in Figure 1 [21]. The investigation of DSI in these species is a convenient model for application of the proposed experimental approach. | ||||||
|
Materials and Methods
| ||||||
|
The DNA sequences of E. coli K12, S. typhimurium, and B. subtilis, obtained from KEGG database (http://www. genome. jp/kegg/kegg2. html) were analyzed. The DNA sequences of 90 genes with direct orientation and 90 genes with reverse orientation were investigated for every species. The number 90 was selected because it is the maximal number of genes with long (200 nucleotides) intergenic sequences in S. typhimurium, and B. subtilis. In E. coli genes with 200 nucleotides intergenic sequences were taken from the beginning of chromosome without any selection. The DNA sequences located in a gene promoter zone from -200 to +200 nucleotides were investigated. The nucleotide composition and the energy of DSI of promoter and coding DNA sequences in direct and reverse orientation were analyzed. For assessment of the energy of DSI in the DNA double helix we assessed two types of interaction between nucleotides: hydrogen bonds and base staking interaction. The energy of interaction due to hydrogen bonds was assumed: T-A -68, 8 kJ/mol and G-C -115, 1 kJ/mol [22]. Values of free energy of coaxial stacking range from -0.85 kcal/mol for TA dinucleotide stack to -2.76 kcal/mol for GC, free energy of base-stacking interactions is presented in (Table 1) [23]. The analogous approach was applied for DNA strands interaction energy oligonucleotides [24]. The impact of stacking energy in the total DSI is relatively small compared to hydrogen-bond energy, but it also take into consideration properties of the neighbouring nucleotides. We calculated the mean energy of DSI per one nucleotide in sequences of 50 nucleotides (spans: -200 to -151; -150 to -101; 100 to -51; 50 to -1 and 1 to 50; 51 to 100; 101 to 150; 151 to 200). We used sliding window with breadth 10 nucleotides and mean DSI energy was summarized for every 50-nucleotide span. The mean data of DSI energy are presented in (Figure 2) (Figure 3) (Figure 4). To show the distribution of energy of DSI along DNA the nucleotide sequence was applied the method of sliding window with 10 nucleotides width. The mean energy of DSI was calculated for 10 nucleotides and was ascribed to the fifth nucleotide in the window. After this we calculated the mean data for 90 sequences (Figure 5) (Figure 6) (Figure 7) (Figure 8). As a result we have obtained the energy distribution along nucleotide sequence for directly and reversely oriented genes separately. To determine the probability of the difference between spans of DNA we applied a statistical processing by t-student and ANOVA tests. The difference between variances with probability level p<0.05 are marked with asterisks (*). | ||||||
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
| ||||||
| ||||||
| ||||||
|
| ||||||
| ||||||
|
Results and Discussion | ||||||
|
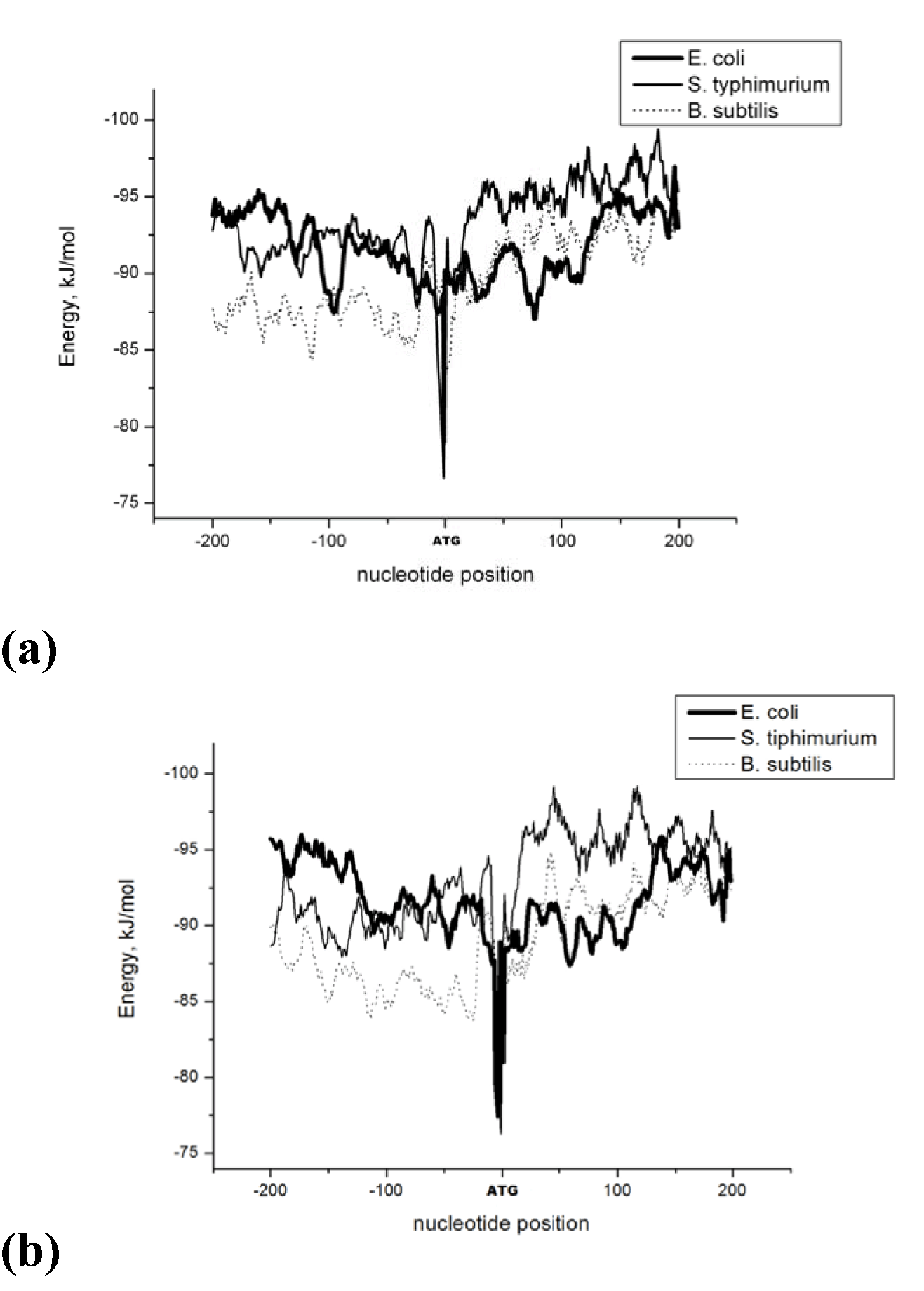
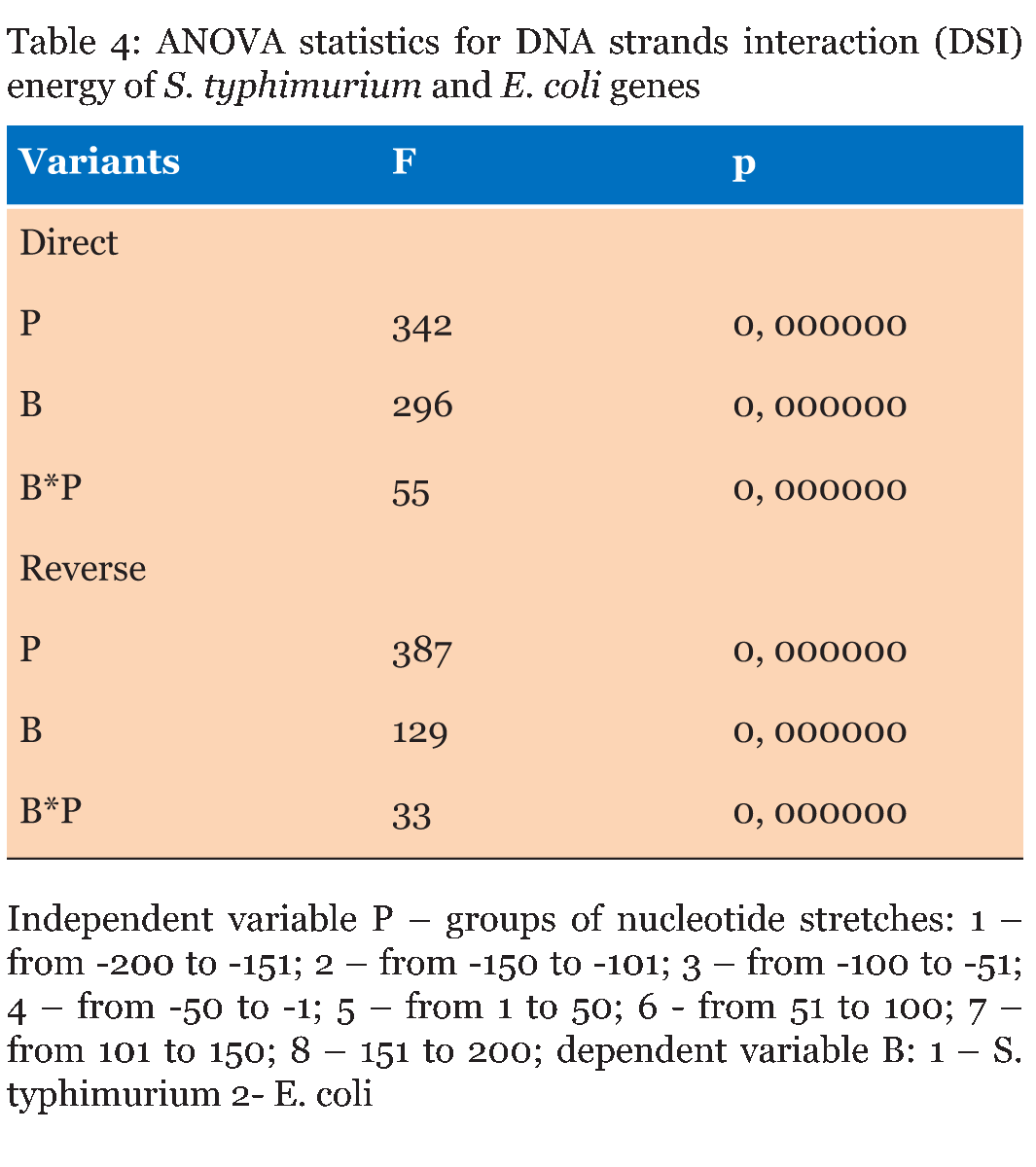
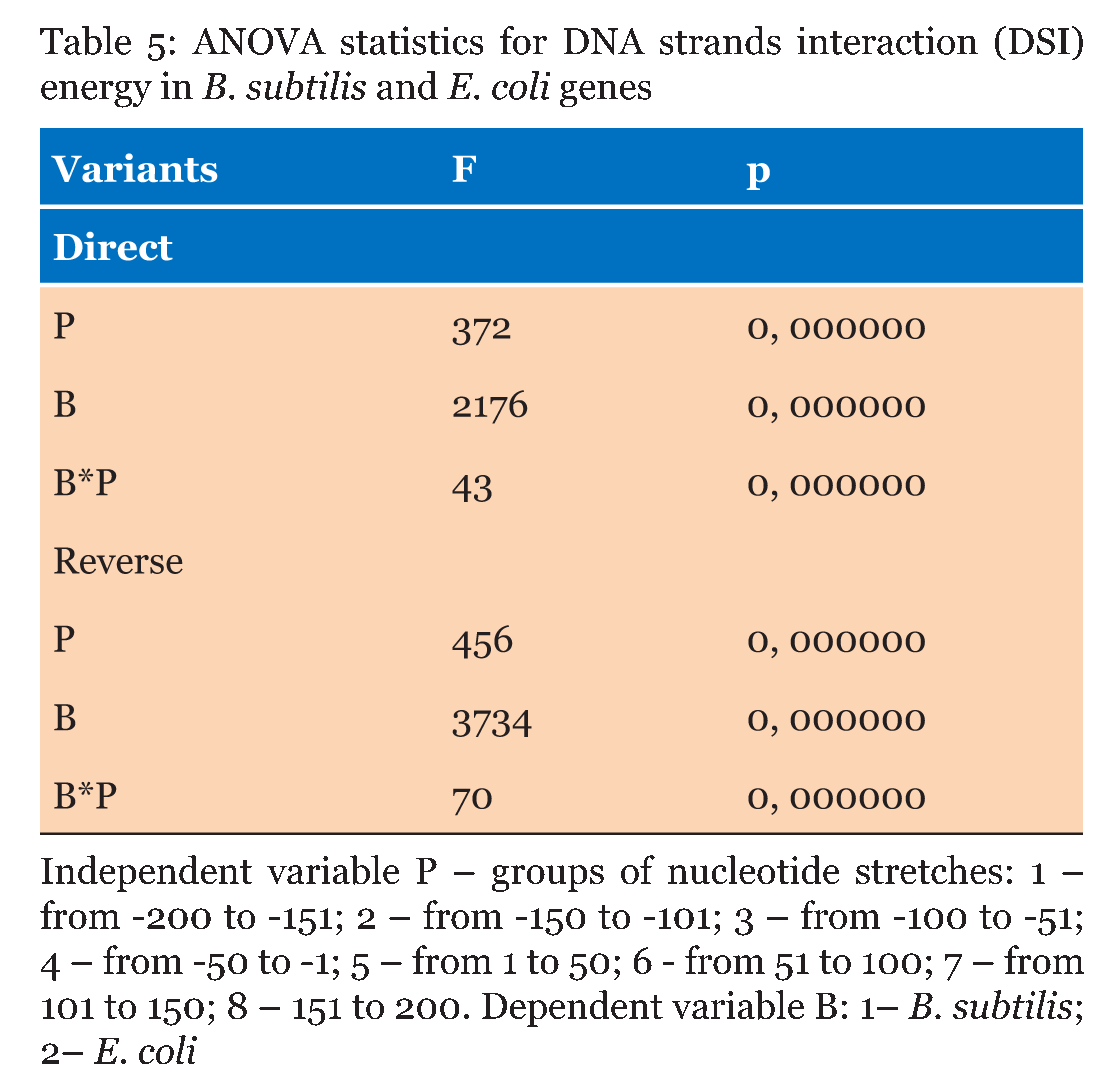
The Energy of Dna Strands Interaction in Escherichia coli Genes The data of DSI energy in E. coli are presented in Figure 5. As one can see, it gradually decreases in the near promoter and promoter area from -150 to +1 nucleotide and it is minimal in promoter area. The data of analysis of DNA strands energy interaction in 50 nucleotides spans are presented in Figure 3. These data indicate a statistically significant decrease of absolute value the energy of interaction of DNA strands in the region from -100 to -1 (Figure 2A) that supports conclusion based on the data of Figure 5. For convenience, we present the absolute value of DSI energy. As one can see, in the coding region the energy of DNA strands is decreased in the spans from 1 to 50 and from 50 to 100 nucleotides for both types of genes. The energy of DSI is lower in genes with direct orientation in spansfrom-200 to - 151, from-100 to -1, and from +50 to +101 regions, but in the region from-150 to -101, from + 1 to +50, and from + 151 to +200 the DSI energy in directly oriented genes is higher. The energy of DNA strands interaction in Salmonella typhimurium genes Figure 6 shows data of analysis of DNA DSI energy of Salmonella typhimurium LT2 genes in the direct and reverse orientation. From the data of Figure 6 it may be seen that the energy of DSI has two disti nct minima (in absolute value) at the -35 nucleotide positions, and around -10, in sites of the binding of regulatory proteins and RNA polymerase. As one can see from Figure 3, in genes of reverse orientation the DSI energy is less in genes with reverse orientation than in genes with direct orientation from -200 to-1 and from +51 to +200, in absolute value. On the contrary, the energy of DNA interaction in genes with direct orientation is less in the zone from -1 to +50, in absolute value. The energy of DNA strands interaction in Bacillus subtilis genes The data of analysis of the energy of DSI in Bacillus subtilis are presented in Figure 4 and Figure 7. The DSI energy is less in prepromoter region than in coding region of gene and minimum of DSI energy is observed in promoter region Figure 7. It the spans from -150 to +1 and from +51 to +150 the genes with direct orientation have higher DSI energy (in absolute value), as it is seen in (Figure 4A-B). In the region from -150 to + 1 the DSI energy is lower (in absolute value) in genes with reverse orientation. The DSI energy of DNA 50 nucleotide stretches was analysed by means of ANOVA statistics. It was shown the significant difference (p>0, 001) in DSI energy of subsequent DNA 50 nucleotide stretches (factor P) in three bacterial species (Table 2). The significant differences in DSI energy were also revealed between three bacterial species (dependent factor B in Table 2). The direction of gene (independent factor D in (Table 2)) also significantly (p=0, 044) influences on the DSI energy. If we compare the DSI energy of 50 nucleotide stretches in bacterial species by pairs: 1 - S. typhimurium - B. subtilis; 2 - S. typhimurium - E. coli; 3 - B. subtilis - E. coli by ANOVA statistical method we can see the significant differences in mean DSI energy between bacterial species. It is true for genes as in direct and also in reverse orientation (Table 3) (Table 4) (Table 5) . The comparative analysis of energy of DNA strands interaction in three bacterial species The DSI energy in genes with direct and reverse orientation for three organisms: Escherichia coli, Salmonella typhimurium, and Bacillus subtilis are presented for genes in direct (Figure 8a) and reverse orientation (Figure 8b). As it can be seen from (Figure 8), showing the mean DSI values in prepromoter region (from -200 to -1) it may be seen that the pattern of energy distribution along DNA is closer in E. coli and S. typhimurium, than in B. subtilis. The mean DSI energy from -200 to -1 in genes with direct and reverse orientation is lower (in absolute value) in genes of B. subtilis, than in genes of E. coli and S. typhimurium (Figure 9). This presumably is connected with relatively distant systematic position of B. subtilis from E. coli and S. typhimurium. The differences in the mean DSI energy between bacterial species may be connected with functional activity ofgenes, because the analysis of DSI revealed DSI energy decrease (in absolute value) near around -55, -35, -10 and +6 nucleotide positions, as it is known, these positions are very important in connection to transcriptional activity regulation [25]. The further investigation of the patterns of DSI energy may be done in connection with the ecologically conditions in which the species exist. Also such investigation may be done in connection with the evolutionary history of species. | ||||||
|
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
|
| ||||||
| ||||||
|
Conclusion
| ||||||
|
| ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions:
Papirny Maxim A. – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Critical revision of the article, Final approval of the version to be published Pasiuga Vladimir N. – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Critical revision of the article, Final approval of the version to be published Shckorbatov Yuriy G. – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Critical revision of the article, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© 2015 Papirny Maxim A. et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|